Fetal and New Born Genetic Medicine can treat genetic conditions in the womb
Genome Editing and the Fetal Environment
Genome editing tools are capable of modulating gene activity without necessitating alterations to the genome itself, and somatic genome editing encompasses interventions that may be implemented prenatally. The advancement of fetal blood transfusions, pioneered in the 1960s to address anemia resulting from RH incompatibility, has paved the way for a diverse array of therapeutic strategies, including stem cell transplantation, enzyme replacement therapy, and the prospective development of fetal genome editing methodologies.
The fetal environment provides several benefits compared to postnatal therapies, such as reduced immune response complications, swift stem cell proliferation, enhanced ability to cross the blood-brain barrier, and the capacity to deliver therapies to various organs. Implementing routine screening for prevalent conditions alongside genome editing could significantly broaden the spectrum of patients and conditions eligible for treatment. One notable challenge in fetal genome editing is the disparity in risks and benefits experienced by the mother and fetus, yet a recent survey found that a significant majority of parents supports the availability of such treatments.
Potential Fetal Therapy: SYNGAP1
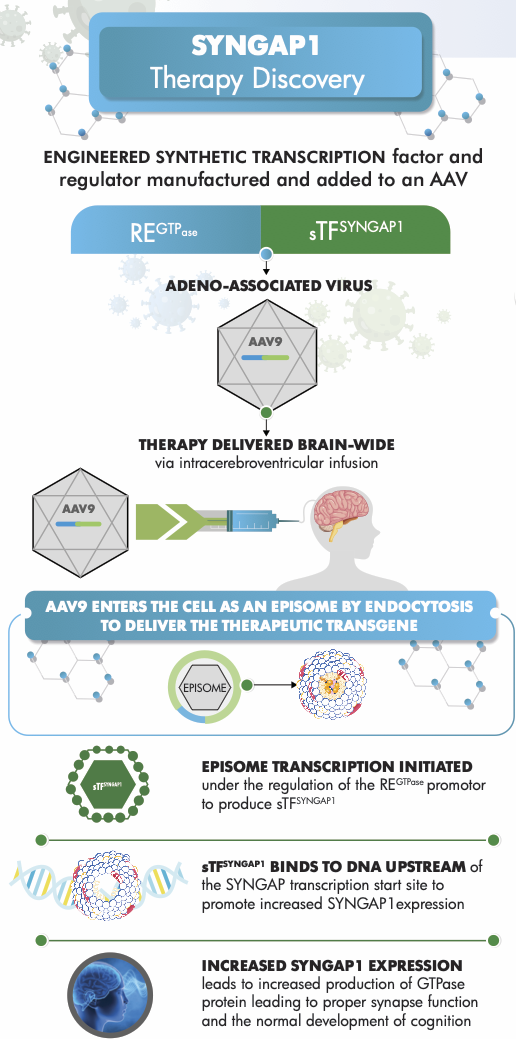
SYNGAP1 is an important genetic factor linked to problems such as global developmental delay, autism spectrum disorder, and epileptic encephalopathy. Changes in this gene that cause loss of function can lead to a neurodevelopmental disorder characterized by difficulties in thinking, challenges with social communication, and seizures that start in early childhood. Research on neurons from mice and rats has demonstrated that Syngap1 plays a crucial role in the structure and function of developing excitatory synapses, where loss-of-function variants result in the formation of larger dendritic spines and increased strength of glutamatergic transmission.
A safe and ethical roadmap
While giving gene therapies before birth may potentially be very effective, we are very cautious about using medical interventions on fetuses. Rather than altering genes, intrauterine protein therapies involve delivering the correct version of the protein that a faulty gene is supposed to make. The priority is to ensure no germline changes are made in a family’s bloodline.
Step-by-Step Guide to Fatal Therapies
Gene Therapy
Cells in the relevant part of the body can have a correct version of the gene introduced or the DNA can be changed using CRISPR “gene editing”
Protein Therapy
Instead of editing DNA, the correct version of the protein encoded by the gene can be given to the fetus. So far this has been done successfully for two medical conditions one that causes a lack of teeth and sweat glands, and a usually fatal metabolic disorder
Stem Cell Therapy
Stem cells without the faulty gene are given to a fetus, with the aim that they endure and become a substitute for the recipient’s cells. So far this has helped several children with brittle bone disease
The services, products and technologies described on this website will be offered on a when-and-if-available basis and is subject to regulatory approvals in all territories. The statements on this website are not intended to be - and should not be interpreted as - a commitment, promise, or legal obligation, and the development, release, and timing of any services, products and technologies described is subject to change, and it remains at the sole discretion of GENURON to deliver or delay the delivery of any of the products, services, or technologies set forth herein. GENURON is a start-up company and statements in this website are forward-looking statements that are subject to considerable risk and uncertainty. Important factors that could cause actual results, products, services or technologies to differ materially include: failure to produce drugs or therapies as expected by our scientists; regulatory rejection of our products or technologies; consumer rejection of our products and technologies; design or manufacturing defects; changes in industry standards; unexpected loss of performance of our products or technologies when used by third parties; and reputational risks. These forward-looking statements are not guarantees of future performance and, except as required by law, GENURON disclaims any obligation to update these forward-looking statements to reflect future events or circumstances.
