Revolutionizing Cancer Treatment with Personalized Vaccines
Personalized cancer vaccines represent a groundbreaking approach in the fight against cancer. By leveraging the unique genetic makeup of each patient, these vaccines stimulate the immune system to recognize and attack cancer cells more effectively. This innovative treatment is a significant advancement in modern oncology, offering hope for improved outcomes and reduced side effects compared to traditional therapies. At Genuron, we are at the forefront of this medical revolution, committed to transforming cancer care through our expertise in generative genomics and biopharma.
Personalized Vaccine Creation
1. Genetic Profiling
Sequence the tumour genome and find all its genetic mutations
2. AI-Driven Vaccine Design
Find which neoantigens are likely to elicit the strongest response from the patient
3. Vaccine Synthesis
RNA vaccine injected into the body that creates antibodies against abnormal proteins, triggering an immune response that targets the tumour
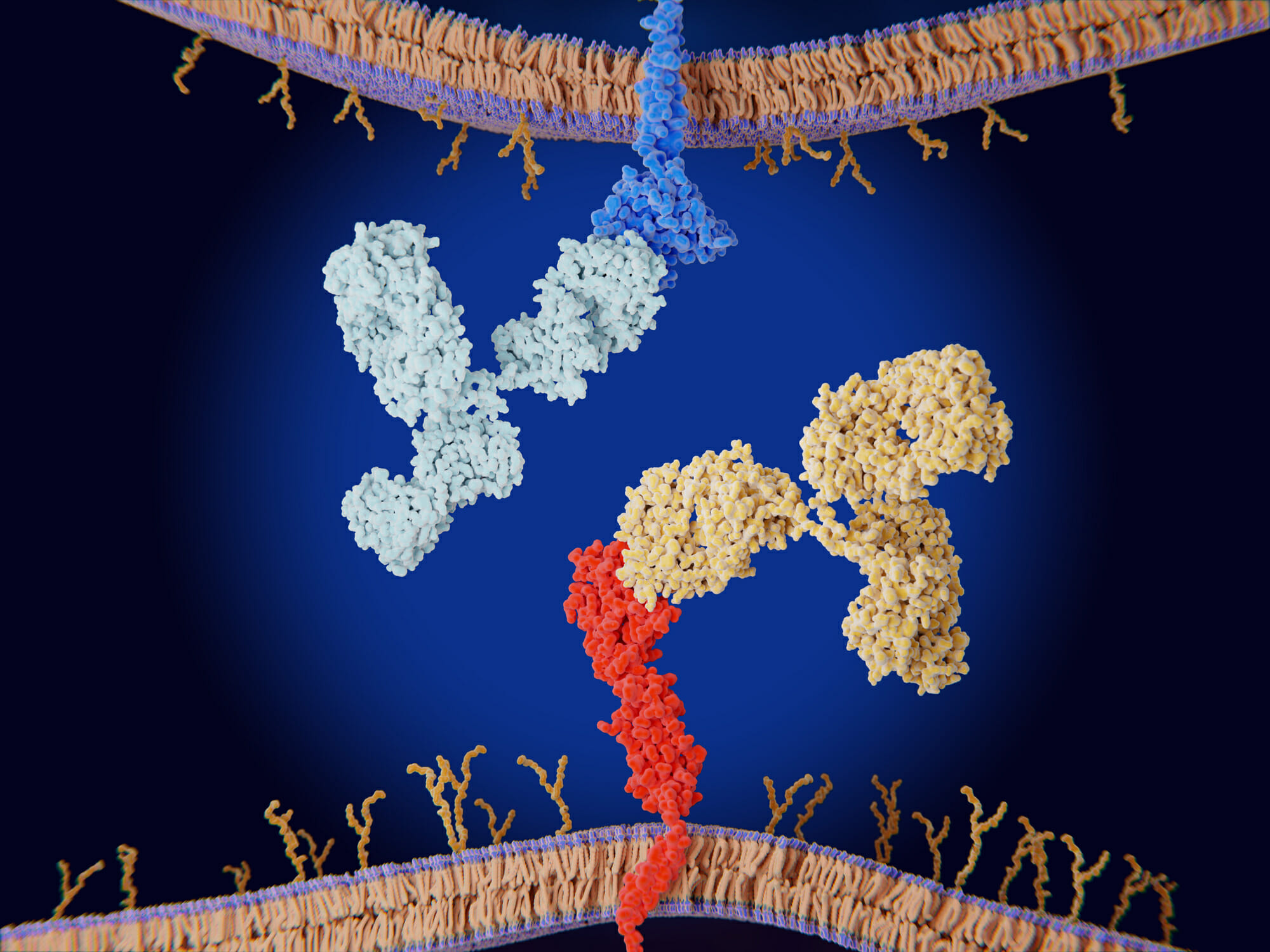
Synergizing with Immune Checkpoint Inhibitors
The potential to reprogram the tumor microenvironment from a cancer-supportive to a cancer-hostile presents innovative avenues for combination therapies. Immune checkpoint inhibitors, particularly anti-PD-1 therapies, have revolutionized the field of oncology; however, their therapeutic effectiveness is often hindered by the immune-suppressive characteristics inherent to the tumor microenvironment. By redefining the tumor microenvironment, we can alter the engagement dynamics, ultimately facilitating enhanced tumor eradication in patients.
Reprogramming cellular senescence in the tumor microenvironment augments cancer immunotherapy
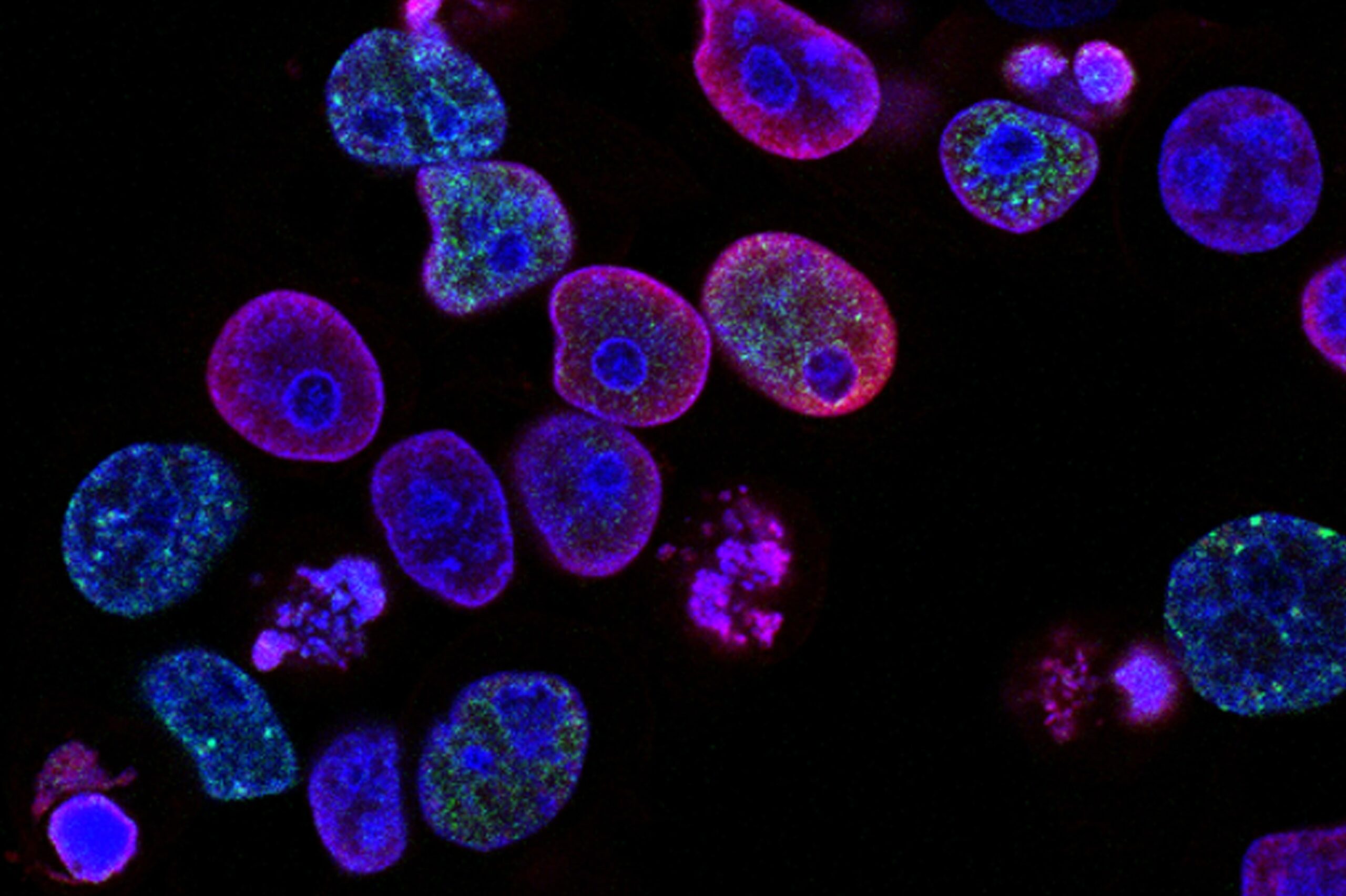
Harnessing the potential of certain aging tumor cells may help us reshape the tumor microenvironment and enhance the body’s immune response against cancer. However, we must handle this potential carefully to prevent strengthening the suppressive effects of these aging cells. Blocking the JAK2/STAT3 pathway can improve the immune response triggered by the Aurora kinase inhibitor alisertib, which leads to a form of cell aging while reducing the negative effects of the senescence-associated secretory phenotype, or SASP, while maintaining its beneficial aspects. By reprogramming SASP using alisertib and the JAK2 inhibitor ruxolitinib this approach to reshaping the tumor microenvironment could become a promising strategy for cancer immunotherapy.
Focusing on Neoantigen Cancer Vaccines
T cells play an essential role in driving antitumour immunity, and the effectiveness of immune checkpoint inhibitors for cancer treatment underscores the potential of enhancing endogenous T cell responses to achieve tumour regression. Despite this, many cancers, particularly those in metastatic stages, still present high mortality rates. Advances in genetic profiling of tumours and the discovery of tumour-specific antigens have paved the way for the development of personalized therapeutic cancer vaccines aimed at targeting mutated tumour antigens, known as neoantigens, to elicit tumour-specific T cells that enhance therapeutic outcomes. A key consideration is optimizing T cell functionality within the tumour microenvironment. In this perspective, we examine neoantigen vaccines and suggest a novel strategy whereby intravenous vaccination, or its combination with tumour-targeting immune modulators, may bolster antitumour efficacy by amplifying the quantity, quality, and diversity of T cells while concurrently converting the tumour microenvironment into a predominantly immunostimulatory landscape conducive to T cell activity.
The services, products and technologies described on this website will be offered on a when-and-if-available basis and is subject to regulatory approvals in all territories. The statements on this website are not intended to be - and should not be interpreted as - a commitment, promise, or legal obligation, and the development, release, and timing of any services, products and technologies described is subject to change, and it remains at the sole discretion of GENURON to deliver or delay the delivery of any of the products, services, or technologies set forth herein. GENURON is a start-up company and statements in this website are forward-looking statements that are subject to considerable risk and uncertainty. Important factors that could cause actual results, products, services or technologies to differ materially include: failure to produce drugs or therapies as expected by our scientists; regulatory rejection of our products or technologies; consumer rejection of our products and technologies; design or manufacturing defects; changes in industry standards; unexpected loss of performance of our products or technologies when used by third parties; and reputational risks. These forward-looking statements are not guarantees of future performance and, except as required by law, GENURON disclaims any obligation to update these forward-looking statements to reflect future events or circumstances.
